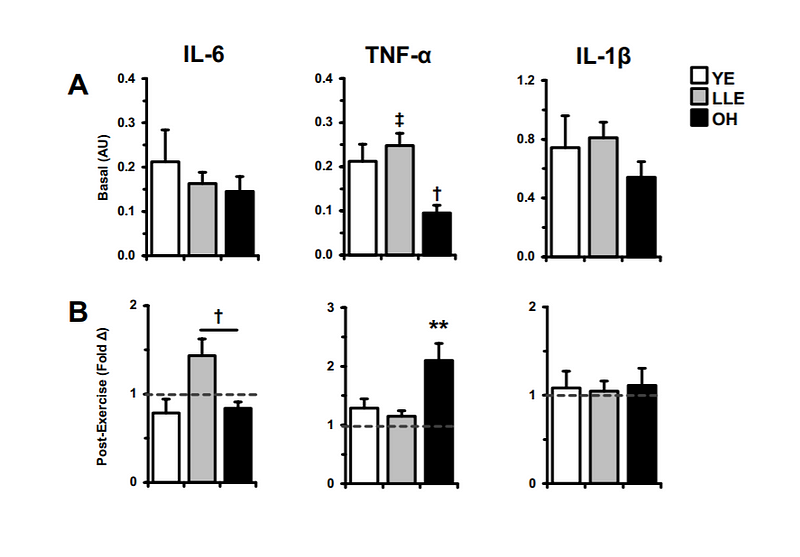
Elite and recreational runners train their bodies to run fast and far. This is typically accomplished through a variety of training stimuli including faster intervals, long runs, and tempo workouts. An effective training program will be designed to enhance three key variables that combine to determine running performance: VO2 max (aerobic capacity), lactate threshold (and speed at lactate threshold), and running economy.
The latter, running economy, is perhaps the variable that can change the most, especially in elite runners. Simply put — running more economically (i.e. using less energy to cover the same amount of distance) means you can run faster for longer.
Training isn’t the only thing that can improve running economy — technology can too. This became apparent when Nike, in 2017, released the first prototype of their “4%” shoe — named so because initial laboratory testing showed that wearing these shoes could save runners about 4% in terms of energy expenditure vs. other shoes.
What makes the shoes so special? Several technological advancements including a carbon-fiber plate with a unique curvature and an extremely thick midsole composed of innovative materials.
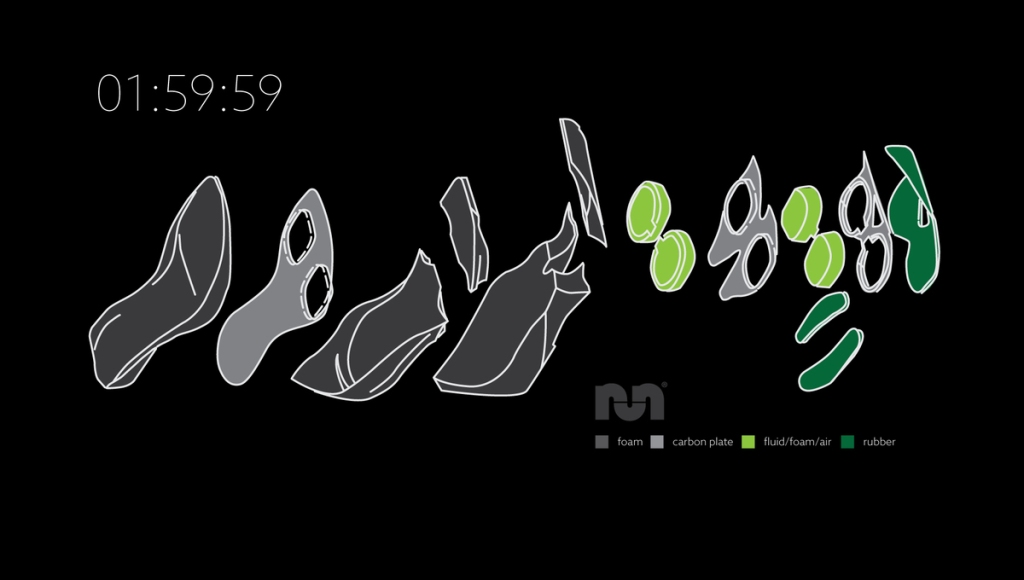
The advent of these shoes has sparked controversy over their “fairness” and legality. Some think they should be banned, or otherwise that world records run in these shoes should be marked with an asterisk. Nonetheless, elite marathon runners (and many non-elites) are sporting the new “supershoes” at races around the world.
All of the hype surrounding the shoes (models which now include the 4%, Next%, VaporFly, and AlphaFly) begs the question — do they actually work?
A new study published in the Journal of Applied Physiology sought to answer this question using real-world marathon race data.
Primary objective
There were a few different aims to this study. First, the authors wanted to determine whether, overall, runners who wore the neoteric Nikes (this is what they call the Nike “supershoes” in the paper) run faster than runners in other shoe models (i.e. non-Nikes).
The authors of this paper also wanted to investigate whether performance improvements (if any) in the shoes were different among male and female marathoners. In addition, they performed a within-runner analysis to see whether a particular runner improved performance when they started wearing the neoteric Nikes.
Brief study methods
Marathon finish times and racing shoes worn by athletes were analyzed for the top 50 male and female finishers at four major marathon races: the Boston Marathon, Chicago Marathon, London Marathon, and New York City Marathon between 2010 and 2019.
Average finish times were compared among runners who wore the neoteric Nikes and those who wore other shoe types. Additionally, where data were available, the change in marathon performance was calculated for runners who ran races with and without the neoteric Nike shoes.
Results
Data were analyzed for 3,886 marathon performances during the period of interest. Here’s what they had to say on the performance effects of “supershoes.”
- In 2019, more runners started wearing neoteric Nikes (66.3% of males and 58.4% of females)
- In 2019, marathon performances were significantly faster when compared to times in 2010-2018 (when fewer runners were wearing Nike “supershoes”)
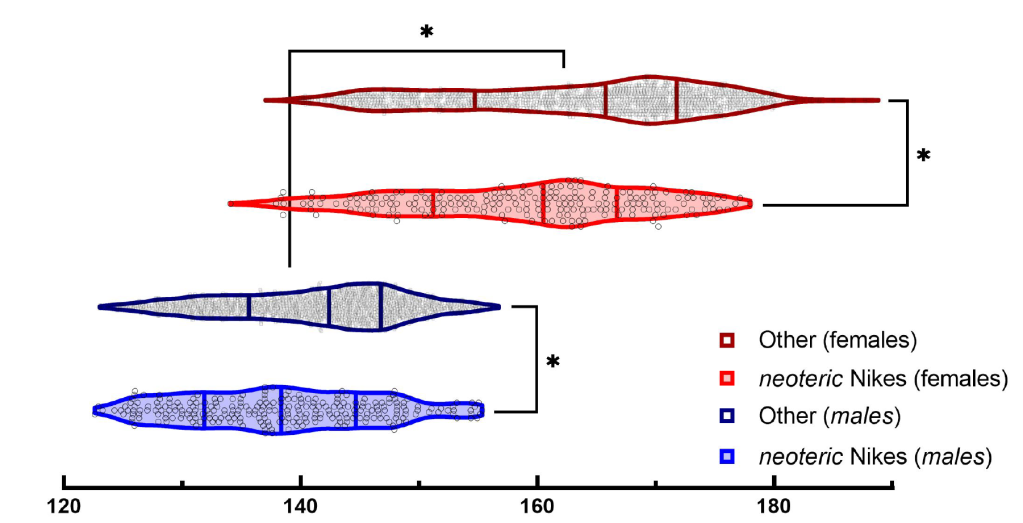
- Marathon times were faster for runners wearing neoteric Nikes vs. other marathon racing shoes, consistent in both males and females
- Males wearing Nike shoes ran ~2% faster (2.8 minutes faster) than non-Nike wearers
- Females wearing Nike shoes ran ~2.6% faster (4.3 minutes faster) than non-Nike wearers
- Females had a greater improvement in marathon performance while wearing Nike shoes compared to males (~2.6% vs. ~2% improvement, respectively)
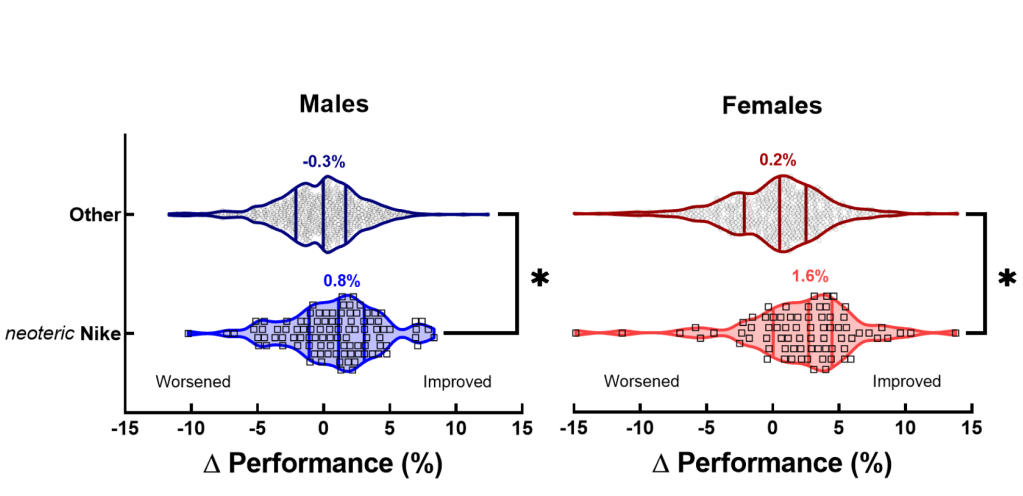
- Changing to neoteric Nike shoes improves performance — the between-race difference for a runner wearing vs. not wearing neoteric Nike shoes was 0.8% for males and 1.6% for females (corresponding to a 1.2 minute improvement for males and 3.7 minute improvement for females)
- The performance advantage of neoteric Nike shoes was consistent across all of the marathon race courses analyzed
These data complement other real-world and laboratory data that confirm the “ergogenic” effect of wearing the technologically-advanced Nike “supershoes.” Don these shoes, evidence says, and you’ll run faster. While the improvements seen here are a bit less than the 4% claim that staked in the shoe’s name, the magnitude is still significant from a statistical and a performance perspective.
An elite runner gaining a 1-5 minute improvement in their marathon time from a shoe — that’s incredible. No extra training, no tweaks to nutrition required; just lace up a different pair of sneakers (those with a swoosh).
It should be noted that Nike is not the only company introducing new shoe technology — nearly all major running brands now have their own version of a carbon fiber-plated racing shoe. It has become a race (pun intended) to see who can design the chunkiest, cushiest, and springiest shoe on the market.
Personally, I think that the debate over whether these shoes should be banned is ludicrous. They clearly improve performance, but in general, most athletes now have access to this once-limited technology, meaning that there is no relative advantage for those who use them.
That being said — why not see how far (and how fast) technology can take us? Why constrain the limits of human performance because a shoe seems “unnatural” or ruins the “purity” of the sport? Advancements — in training, nutrition, psychology — are what allow us to redefine what is possible in the world of athletics. The way that I see it, shoe technology is just another aspect of natural progression in athletics, and we should enjoy the trajectory in which the sport is headed.
Study cited