Why do we sleep? This question has puzzled researchers, philosophers, and crying babies for centuries. I love sleep as much as anyone. But, personally, if I could, I’d love for someone to figure out a way to remove this evolutionary drive and grant me the extra 1/3 of my life that I’m losing every night.
Alas, the inevitability that everyone needs sleep leads us to study the reasons for it, to understand this odd phenomenon. There are several evolutionary, physiological, and regulatory reasons proposed. Blood pressure dips at night during sleep, which is probably a way that we preserve some of our cardiovascular function and prevent damage. Muscles repair and hormones are expressed or reduced — each of these functions is also necessary for long-term maintenance of our body.
In particular, the effects of sleep on the brain seem to be some of the most important. Sleep is necessary to cement memories and enhance the learning of facts and skills. The newly discovered glymphatic system uncovers a new role for sleep in removing certain proteins, fluids, and harmful molecules from the brain — a functional waste clearance system for the brain. Dysfunction of this system (in sleep disorders) is proposed to contribute to central nervous system disorders, cognitive decline, and neurodegeneration.
A new study uncovers another novel role for sleep in the brain in which is may facilitate the repair of DNA at the level of neurons. This process is undoubtedly necessary for the proper short- and long-term function of organisms and perhaps essential to maintaining cognitive integrity. While the study was in Zebrafish, the implications for humans are vast and interesting to discuss.
Fortunately, I had the chance to co-host a podcast with Dr. Kevin Folta from the University of Florida in which we interview Dr. Lior Applebaum, lead researcher on the study titled “Sleep increases chromosomal dynamics to enable reduction of accumulating DNA damage in single neurons.”
Check out the podcast here.

As a necessary primer, we first ask the question, “what is this thing we call sleep?”
This seems obvious but surprisingly, science isn’t completely sold on a definition of sleep from a physiological and functional perspective.
All animals have to sleep, the question is why?” Sleep is a very dangerous behavior, Applebaum says, and not very good for survival—during this time we are exposed to predators, very vulnerable to predation, so it doesn’t make sense in terms of survival. This suggests it is essential for the brain and behavior.
Here, I’d like to use a quote from Dr. Matthew Walker, author of “Why We Sleep” who has said…
“If sleep doesn’t serve an absolutely vital set of functions, it’s the biggest mistake the evolutionary process has ever made.”
Another question is, outside of single neurons, why is sleep important and at the neuron level, what function does it serve. Furthermore, what happens when we are deprived of sleep?
This might seem like another obvious question and answer, but we don’t really have all of the information needed to tell us what happens in cells to explain what essential functions sleep plays.
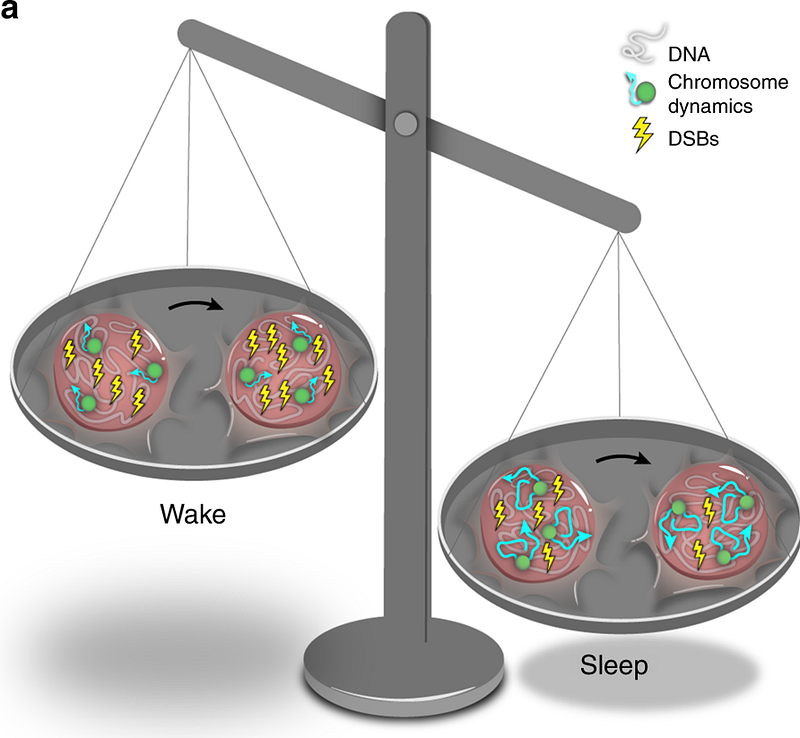
Professor Applebaum states that, during wakefulness, our brain (neurons) accumulate DNA damage This damage isn’t really dangerous, it’s just a natural cell process involving double strand breaks (dsbs) in DNA. In neurons during wakefulness, damage accumulates.
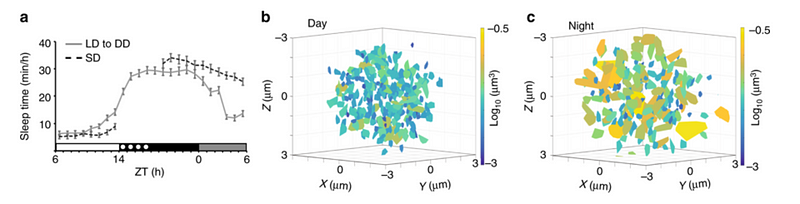
If we sleep deprive (fish, in this specific study) MORE damage accumulates, and only when the organism goes to sleep does the DNA damage get reduced and return to normal. What are the mechanisms behind this? During waking hours, chromosome dynamics (a marker of repair activity) are low. During sleep, Applebaum’s team observed that neurons increase chromosome dynamics two-fold.
This is interesting, since the thought is that during sleep, things “calm down” and activity is reduced. While true for some systems, this study suggests that the opposite might be true in neurons — certain activities increase during sleep.
Increased chromosome dynamics are necessary for repair. When we deprive people (or fish) of sleep, chromosome dynamics stay low all the time and the necessary repair won’t occur.
Alright, well, we aren’t Zebrafish. How is sleep different between Zebra fish and birds/other animals and humans.
We base sleep on mostly behavior criteria. For humans, this means it occurs (usually) at night, on a bed, where the arousal threshold is reduced. These features are used to define sleep. Another criteria to define sleep is electroencephalography (EEG) activity. The electrical pattern in the brain is different during wakefulness and different sleep stages. This pattern is unique to mammals and birds and maybe certain reptile species, but isn’t detected in Zebra fish/invertebrates (non-mammalian).
Basically, the electrical activity in fish and other non-mammals is the same during sleep and wakefulness, and can’t be used to define sleep.
In these species, sleep is defined ONLY by behavior criteria. This is one reason Applebaum and colleagues decided to conduct the study. He stated that “maybe we can define sleep using this cellular marker of increased chromosome dynamics.”
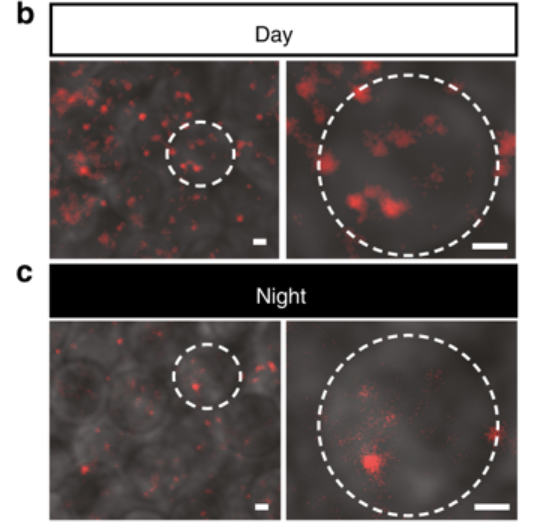
Measuring DNA repair in neurons seems difficult (and probably is). Using specific techniques, Applebaum’s team was able to visualize and quantify the amount of DNA activity and chromosomal repair during sleep. To do this, they tagged DNA in the brain with fluorescent proteins (they glow!) which can then be visualized for their movement and activity.
The ability to visualize moving chromosome inside a single neuron inside a live animal is unique to the zebrafish. Once the fluorescent proteins are taken and bound to proteins that interact with DNA, what you get is a glowing dot (puncta) inside the cell. Then, researchers can track the movement in real time and make a movie of the dots.
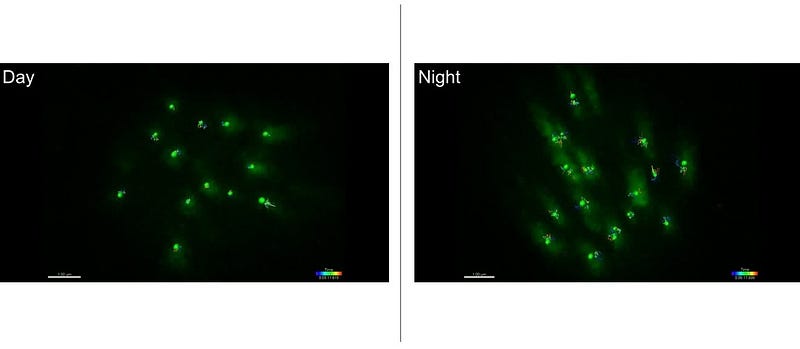
Since the researchers can monitor chromosome activity all the time, they are able to determine when the least and most amount of activity occurs.
“Repair happens all the time, even during wakefulness, otherwise cells would be at risk for disease and death/cancer, etc.,” Applebaum states. He goes on to explain that findings from the study indicated that during sleep, repair is more efficient.
Efficient in this case means that it’s working harder or better because we have less damage occurring. What is quantified is the amount of DNA damage or sites of damage in a single cell during the day and night.
Applebaum provides an excellent analogy to describe the repair and cleaning mechanisms that occurs during sleep.
He compares is to a “city with a lot of traffic and cars during the day.” A lot of road damage is accumulated, trash is everywhere. You can clean the roads during rush hour but it wouldn’t be efficient, since more cars and more trash would still come to damage and litter the roads. But, at night when the city isn’t busy with wakefulness (cars), the trucks that repair the road and remove the trash can move faster and do the repair faster and more efficiently.
This is how the chromosome work. The energy usually dedicated to wakefulness can now be used during sleep by cells for nuclear and cellular maintenance.
We know that chromosome dynamics = repair. But what does the term “chromosome dynamics” actually mean?
Chromosome dynamics is basically “the volume of the movement of each chromosome. The DNA is moving all the time inside each cell — repairing, transcribing DNA, etc. Dynamics is the volume of movement at any one time; which during the day is low, and which during night is high.”
Many people use sleeping pills or melatonin to sleep. Some studies suggest the quality of sleep induced “artificially” isn’t the same as natural sleep. When it comes to dynamics and repair, it turns out that we might get the same type of benefit whether our slumber comes naturally or synthetically; and whether it occurs at night or during the day.
“Fish are highly sensitive to melatonin”, Appleaum explains. In this study, “it was shown that melatonin is a strong sleep promoting hormone in Zebrafish. If you give [Zebrafish} melatonin during the day they fall asleep within a minute or less.”
While they aren’t completely sure about the quality of sleep, during the day when sleep is induced by melatonin, chromosome dynamics increase in a similar manner to natural sleep. This suggests that the DNA repair is unique to sleep, and not dependent on circadian rhythms.
DNA damage occurs in all cells — the primary example is the damage induced by UV rays (the sun) to the skin. This begs the question of whether or not this repair process during sleep is unique to neurons, if it exists in other cell types, and if sleep is necessary for DNA repair in these other cells.
The study also tested DNA damage and chromosome dynamics in glial cells (also in neurons) and endothelial cells (in blood vessels). Interestingly, both cell types experienced chromosome dynamics and DNA damage but did not express any differences between day and night or wake and sleep.
This suggests that damage and repair is happening all the time in these cells. They don’t require sleep or a long offline period for repair to occur — but instead undergo a constant process of damage and repair. The group hopes to this in other cells like heart and muscle cells in future studies.
Why do neurons accumulate damage during waking times? Sure, we are all exposed to toxins, pollutants, and other harmful stimuli, but what is the main source of DNA damage in the brain?
“All cells accumulate DNA damage for different reasons. What is unique to neurons is that even neuronal activity itself causes double strand breaks — normal activity.
“I think, therefore I…induce DNA damage?”
This “damage” isn’t harmful to the cell, but allows more efficient gene expression, Applebaum says. But “at some point you have to make sure you return everything to normal.”
Applebaum also provides data from the study indicating that if you artifically induce DNA damage (using chemicals) or sleep deprive the zebrafish, you can actually cause them to fall asleep.
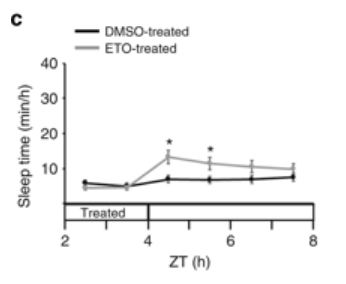
This is evidence that sleep is governed by some sort of “homeostatic pressure” Accumulating double strand breaks in Zebrafish triggers sleep. If you sleep deprive fish, they go to sleep immediately after and sleep more in the following day (sleep rebound) until they finish repair.
Even Zebrafish like to sleep in on the weekends, it seems.
From this fascinating talk on a topic I’m increasingly becoming interested in, I had many takeaways but mainly, my suspicions were confirmed.
Sleep is vitally important is important. Often times, we used “tiredness” as a scale to determine whether we need sleep, have slept enough, or can continue to go without sleep. What studies like this one indicate is that there are some pretty damn important cellular changes happening during sleep that we can’t detect, and which might only manifest later down the road.
Several neurodegenerative diseases are associated with poor sleep. The evidence that sleep is necessary for proper brain repair supports this association.
You might not think you need the recommended 7–9 hours, but unless you’re one of those lucky DEC 2 gene mutants who have a genetic predisposition to need less sleep than other humans — you (and your brain) probably do.